rcprd: An R package to simplify the extraction and processing of CPRD data, and create analysis-ready datasets
rcprd.RmdThis article does not contain any real patient data. All patient data has been simulated but formatted to match the structure of CPRD Aurum data.
1 Introduction
The Clinical Practice Research Datalink (CPRD) is a large resource of Electronic Health Records from the UK, owned by the UK Medicines and Healthcare products Regulatory Agency (MHRA), and containing information on demography, medical history, test results and drug use of individuals registered with a general practice. The MHRA maintain two databases, CPRD GOLD, (Herrett et al. 2015) which contains data from general practices using the Vision computer system, and CPRD Aurum (Wolf et al. 2019), which contains data from general practices using the Egton Medical Information Systems (EMIS) computer system, EMIS Web. The primary care data is linked to hospital data, death registration data, cancer registry data, deprivation data and mental health services data, enabled by NHS digital.(Padmanabhan et al. 2019) As of 2016, the EMIS Web computer system was used by 4199 (56%) of the general practices in England.(Kontopantelis et al. 2018) As of September 2024, CPRD Aurum contained data on 47 million (16 million currently registered) individuals from 1,784 (1,596 currently contributing) general practices across the United Kingdom,(CPRD 2024a) and CPRD GOLD contained data on 21 million (2.9 million currently registered) individuals.(CPRD 2024b) CPRD is a widely used resource: since 2019, a PubMed search reveals there have been 540 studies published which contain “CPRD” in the title or abstract. Extraction of CPRD data and transformation into a format ready for statistical analysis is computationally demanding and requires a significant amount of work. There is limited published software available to aid researchers in the extraction and processing of CPRD data.Yimer et al. (2021)
rEHR (Springate et al. 2017) is an R package for manipulating and analysing electronic health record data, which works by creating an SQLite database on a fixed storage device (i.e. a disk drive), which is then subsequently queried to extract relevant information, faster than with conventional statistical analysis software. rEHR was designed to be database agnostic, and contains functionality for longitudinal data extraction, cutting data by time-varying covariates, matching controls to cases, converting the units of test data, and creating clinical code lists. rEHR is however no longer maintained, it works with an older version of R (3.3.2) and has been archived on CRAN. The aurumpipeline package (The Health Foundation Analytics Lab 2021) contains functions to clean and process CPRD Aurum data, which works by storing the data as parquet files on the disk drive, which are then subsequently queried to extract relevant data. However, aurumpipeline is not available on CRAN and is not provided with any reproducible examples. The R package drugprepr (Yimer et al. 2021) implements the algorithm of Pye et al. (2018) for preparing drug exposure data extracted from CPRD, however it does not deal with the initial data extraction and storing of data.
Given the many studies using CPRD data, and the limited availability software for data processing, this indicates that a large amount of research time is being spent duplicating the work of others in order to extract CPRD data. This study introduces rcprd, an R package designed to assist researchers in working with CPRD Aurum data and creating datasets which are ‘analysis-ready’. The main problem when working with CPRD Aurum data is the size of the raw data. Data on over 47 million individuals results in thousands of raw .txt files, and Terabytes of data, which can be cumbersome to work with. This is a particular issue for R users, as its infeasible to read all this data into the R workspace simultaneously, as R operates using physical memory (RAM). As suggested by Springate et al. (2017), rcprd bypassess this problem by creating an SQLite database which can then be queried for data of interest in order to build an analysis-ready dataset. rcprd then simplifies the process of querying the SQLite database with functions to extract variables such as “most recent test result”, “time until first event”, or “history of a specified condition”. The SQLite database must be created on a secure device or server which aligns with the data storage requirements of CPRD.
We start by discussing the structure of CPRD Aurum data and the approach taken by rcprd for processing this data, which draws heavily on the work of Springate et al. (2017). We then run through a worked example to showcase the functionality of rcprd, which has two main groups of functions. The first are to extract and store the data in a consistent manner. The second group is to query this data to extract patient level variables. We focus on CPRD Aurum, as opposed to CPRD GOLD, given there has been a considerable drop in the number of practices utilising Vision software in the last 10 years, limiting the research utility of the CPRD GOLD database. However, the rcprd package can also be used to manage linked secondary care (HES) and ONS death data, and is flexible to the point that it could be used to extract and store data from any electronic health record, which will be touched on in the discussion.
2 Data Structure and Extraction Process
2.1 Structure of CPRD Aurum data
We first define the terminology which will be used throughout this article:
- Raw data: The raw data provided to the user by CPRD.
- Cohort: A cohort of individuals that meet the inclusion/exclusion criteria for a given research question. In this setting, the cohort is ultimately a vector of patient id’s.
- Analysis-ready dataset: A data frame to which statistical models can be fitted, with one row for each individual in the cohort, and a column for each variable of interest, for example, age at cohort entry, or most recent BMI score prior to cohort entry. For longitudinal analyses, such data frames can be concatenated, with a variable indicating the time point at which the data was extracted.
The raw CPRD Aurum data is split into eight different file types: Consultation, DrugIssue, Observation, Patient, Practice, Problem, Referral, Staff. The data specification is available here: (CPRD 2022). For most research questions, the relevant files are Patient, Observation and DrugIssue. The Patient file contains information about registration into the database, date of death or lost to follow up, year of birth and gender. This file will be required to define a cohort. The observation file contains all medical diagnoses and tests, while DrugIssue contains information on prescriptions. Medical observations are identified by their medcodeid, whereas prescriptions are identified through their prodcodeid.
In order to facilitate data transfer, this data is commonly split by CPRD into numerous smaller files. The different patient files are denoted by the string set1, set2, set3 in the file name. Individuals in the same patient file will have the corresponding string (setX) in the files containing their medical or prescription data. However, there will be more than one Observation and DrugIssue file corresponding to each patient file. For example, the observation files for patients in set1, will have set1 in their file name, and then an extra suffix 1, 2, 3, etc. The same is true for the DrugIssue files. The naming structure for these is as follows:
- aurum_allpatid_set_extract_patient_001.txt
- aurum_allpatid_set_extract_observation_0.txt
- aurum_allpatid_set_extract_drugissue_0.txt
where and . Note that the prefix to the file names may vary (i.e. the ‘aurum_allpatid’ part) however we expect the naming convention with regards to ‘set’, file type, and ‘0’ to remain consistent. If this changes in the future, we will endeavour to update the rcprd as soon as possible.
2.2 Recommended process for extraction
Our recommended process for developing an analysis-ready dataset is as follows:
- Step 1: Extract initial cohort using extract_cohort() and apply initial inclusion/exclusion criteria which can be applied using only the patient file.
- Q1: Do the inclusion/exclusion criteria depend on primary care data?
- If yes -> (proceed to step 2)
- If no -> (proceed to Q2)
- Step 2: For patients that meet inclusion/exclusion criteria from step 1, add relevant primary care data into an SQLite database using cprd_extract().
- Step 3: Query SQLite database in order to apply further inclusion/exclusion criteria.
- Q2: Do the inclusion/exclusion criteria depend on linked data?
- If yes -> (proceed to step 4)
- If no -> (proceed to step 5)
- Step 4: Request type 1 linked data for individuals and apply remaining inclusion/exclusion criteria.
- Step 5: For patients in the final cohort, add relevant primary care data into an SQLite database using cprd_extract(). If an SQLite database was already created in step 2, and only a small number of individuals were excluded in steps 3 and 4, consider skipping this step. However, if a large number of individuals were excluded, it is worthwhile to create the new SQLite database, as it will be much smaller and future queries for extracting variables will run much quicker.
- Step 6: Query this SQLite database for specific codes and tests to create variables for each individual in the cohort. These are stored as .rds objects, which R data analysts will be familiar with.
- Step 7: Combine extracted variables into an analysis-ready dataset, also stored as an .rds object.
type 1 linked data is defined as “linked data required in order to finalise the study population”
This process aligns with the process implemented by CPRD when cohort inclusion/exclusion criteria are dependent on linked data (see Q2 and step 4). The process can be done entirely within R using rcprd functions, without any specialist understanding of SQLite databases. We recommend this process because once set up, querying the SQLite database is computationally much quicker than reading each of the raw files into the R workspace and querying these separately. It also reduces the probability of errors induced from creating numerous loops through the raw data files. We now move onto a worked example, where we showcase how to implement the above process using rcprd and the functions which are detailed in Table 1.
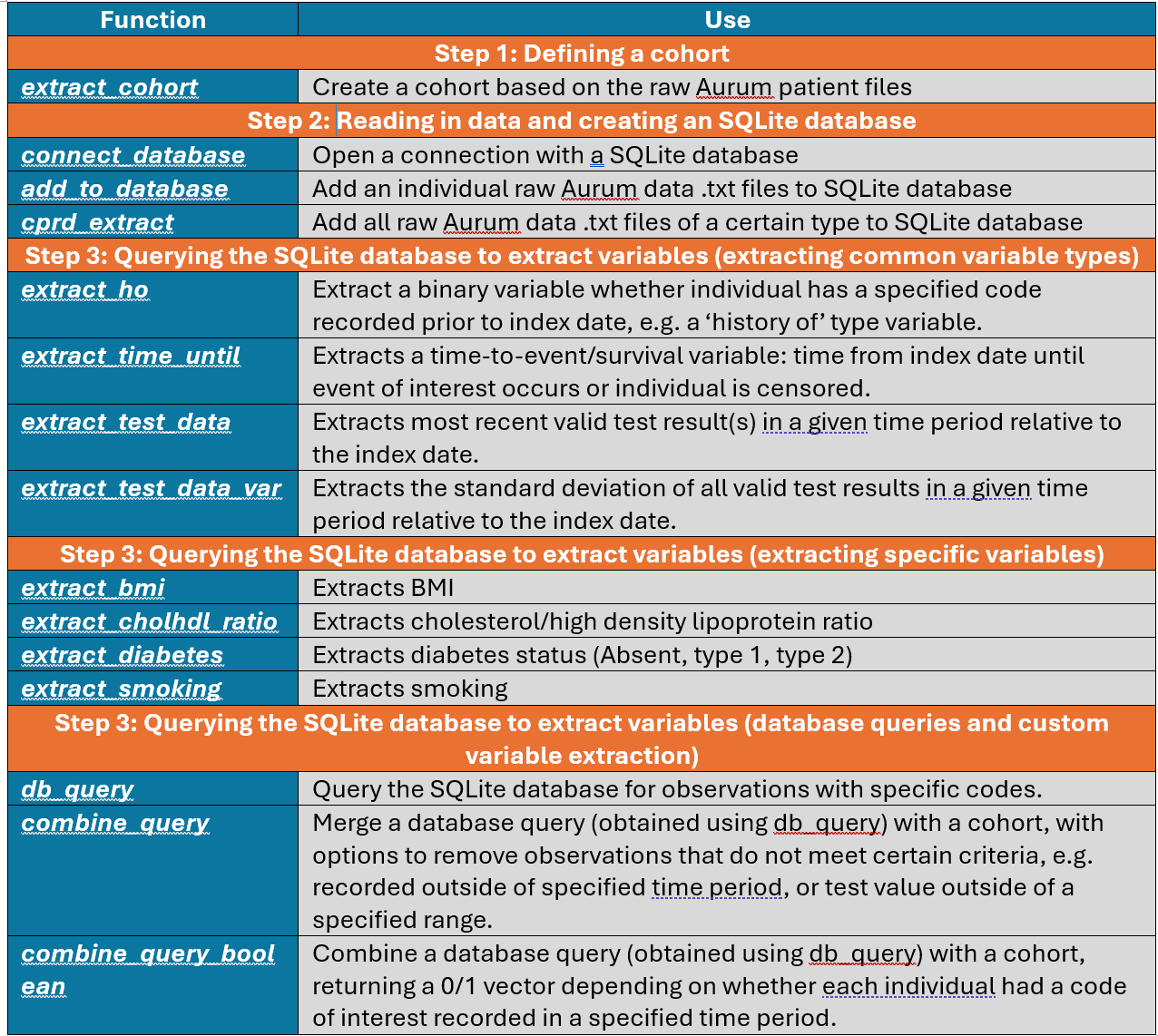
Table 1: Table of rcprd functions
3 Worked example for data extraction
3.1 Steps 1 - 4: Defining a cohort
We have provided simulated patient, observation and drugissue files
which will be utilisied in the worked example. The names of the files
share the same naming convention given in section 2.1, and column names
of the data match the real Aurum data. Numeric variables were simulated
at random as integers between 1 and 100, date variables as a date
between 01/01/1900 and 01/01/2000, gender as an integer 1 or 2, and year
of birth as an integer between 1900 and 2000. Patient id and practice id
were assigned manually. These files are contained in the
inst/aurum_data directory of rcprd. After
installing rcprd, this directory can be accessed using
the command system.file("aurum_data", package = "rcprd").
This contains data on 12 fake patients, split across two patient files
(set1 and set2) and three observation and drugissue
files (all set1):
#devtools::install_github("alexpate30/rcprd")
#install.packages("rcprd") NOT YET ON CRAN
library(rcprd)
#devtools::load_all()
list.files(system.file("aurum_data", package = "rcprd"), pattern = ".txt")
#> [1] "aurum_allpatid_extract_practice_001.txt"
#> [2] "aurum_allpatid_extract_practice_002.txt"
#> [3] "aurum_allpatid_set1_extract_drugissue_001.txt"
#> [4] "aurum_allpatid_set1_extract_drugissue_002.txt"
#> [5] "aurum_allpatid_set1_extract_drugissue_003.txt"
#> [6] "aurum_allpatid_set1_extract_observation_001.txt"
#> [7] "aurum_allpatid_set1_extract_observation_002.txt"
#> [8] "aurum_allpatid_set1_extract_observation_003.txt"
#> [9] "aurum_allpatid_set1_extract_patient_001.txt"
#> [10] "aurum_allpatid_set2_extract_patient_001.txt"The first step in most analyses is creating and defining a cohort of
individuals, which will involve working with the patient files. Data
from the patient files can be combined using the
extract_cohort() function. This will look in the directory
specified through the filepath argument, for any file
containing “patient” in the file name. All files will be read in and
concatenated into a single dataset. In some circumstances, researchers
may be provided with a list of patids which meet their
inclusion/exclusion criteria. In this case, these can be specified
through the patids argument (which requires a character
vector). Suppose the individuals meeting the exclusion criteria are
those with patid = 1, 3, 4 and 6. We would then specify:
pat <- extract_cohort(filepath = system.file("aurum_data", package = "rcprd"), patids = as.character(c(1,3,4,6)))
str(pat)
#> 'data.frame': 4 obs. of 12 variables:
#> $ patid : chr "1" "3" "4" "6"
#> $ pracid : int 49 98 53 54
#> $ usualgpstaffid: chr "6" "43" "72" "11"
#> $ gender : int 2 1 2 1
#> $ yob : int 1984 1930 1915 1914
#> $ mob : int NA NA NA NA
#> $ emis_ddate : Date, format: "1976-11-21" "1972-06-01" ...
#> $ regstartdate : Date, format: "1940-07-24" "1913-07-02" ...
#> $ patienttypeid : int 58 81 10 85
#> $ regenddate : Date, format: "1996-08-25" "1997-04-24" ...
#> $ acceptable : int 1 1 0 1
#> $ cprd_ddate : Date, format: "1935-03-17" "1912-04-27" ...In other circumstances, a user may need to apply the inclusion and exclusion criteria themselves. In this case, one would initially create a patient file for all individuals.
pat <- extract_cohort(filepath = system.file("aurum_data", package = "rcprd"))
str(pat)
#> 'data.frame': 12 obs. of 12 variables:
#> $ patid : chr "1" "2" "3" "4" ...
#> $ pracid : int 49 79 98 53 62 54 49 79 98 53 ...
#> $ usualgpstaffid: chr "6" "11" "43" "72" ...
#> $ gender : int 2 1 1 2 2 1 2 1 1 2 ...
#> $ yob : int 1984 1932 1930 1915 1916 1914 1984 1932 1930 1915 ...
#> $ mob : int NA NA NA NA NA NA NA NA NA NA ...
#> $ emis_ddate : Date, format: "1976-11-21" "1979-02-14" ...
#> $ regstartdate : Date, format: "1940-07-24" "1929-02-23" ...
#> $ patienttypeid : int 58 21 81 10 45 85 58 21 81 10 ...
#> $ regenddate : Date, format: "1996-08-25" "1945-03-19" ...
#> $ acceptable : int 1 0 1 0 0 1 1 0 1 0 ...
#> $ cprd_ddate : Date, format: "1935-03-17" "1932-02-05" ...Often this then needs to be merged with the practice file, which
contains information such as the last date of data collection for the
practice. The practice files can be read in and combined using the
function extract_practices(),
prac <- extract_practices(filepath = system.file("aurum_data", package = "rcprd"))
str(prac)
#> 'data.frame': 6 obs. of 4 variables:
#> $ pracid: int 49 53 54 62 79 98
#> $ lcd : Date, format: "1941-09-20" "1978-01-31" ...
#> $ uts : Date, format: NA NA ...
#> $ region: int 48 51 54 9 7 48and should then be merged with the patient file based on the
pracid variable:
pat <- merge(pat, prac, by.x = "pracid", by.y = "pracid")The cohort of individuals would then be defined by applying study specific inclusion/exclusion criteria. For example, all individuals with > 1 day valid follow up aged 65+, after 1st January 2000. Such criteria can be applied solely using the information available in patient files. In this example, we define the individuals that met the inclusion criteria to be those with patid = 1, 3, 4 and 6.
Once the cohort has been defined, we recommended saving this as an .rds file so it can be easily accessed, as it will be required as input for many of the subsequent functions. In this hypothetical example the inclusion/exclusion criteria are not dependent on linked data or primary care data, and therefore we jump to step 5. However, if the answer to Q1 was yes, resulting in the implementation of step 2, the process for creating the SQLite database (step 2) and querying this to apply the inclusnio/exclusion criteria (step 3) will follow the exact same processes as outlined in sections 3.2 and 3.3. If the answer to Q2 was yes, and type 1 linked data is required to apply the inclusion/exclusion criteria, this can be done without the use of rcprd or an SQLite database. Type 1 linked data is normally provided as a single text file, which can be readily loaded into R and manipulated in order to apply the criteria.
3.2 Step 5: Reading in data and creating an SQLite database
Step 5 reads in the medical/prescription data from the raw .txt files and adds this into a SQLite database. This will only be done for individuals meeting the inclusion/exclusion criteria. The SQLite database is stored on a fixed storage device and will be subsequently queried when extracting variables to create an analysis-ready dataset.
3.2.1 Add individual
files to SQLite database using add_to_database
The function add_to_database can be used to add
individual files to the SQLite database. Start by defining and
connecting to your SQLite database. In this article we create a
temporary database, but in practice this would be a permanent storage
location. Specifically, file.path(tempdir(), "temp.sqlite")
would be replaced by the desired file path and SQLite database name.
aurum_extract <- connect_database(file.path(tempdir(), "temp.sqlite"))Next, we add medical diagnoses data from the observation files to
this database using the add_to_database function. It is
imperative that when adding raw CPRD data to an SQLite database, that
the SQLite database itself is stored in a secure environment which
aligns with the data storage requirements of CPRD. The simulated raw
data provided with rcprd can be accessed using the
system.file function. The vector of patient id’s that
defines the cohort is defined through the subset_patids
argument. Only data with patid’s matching this argument will be added to
the SQLite database. The filetype argument will select an
appropriate function for reading in the .txt files, and also defines the
name of the table in the SQLite database that the files are added to.
Note that for the first file, overwrite = TRUE is specified
to create a new table. For the second and third file,
append = TRUE is specified to append to an existing
table.
add_to_database(filepath = system.file("aurum_data", "aurum_allpatid_set1_extract_observation_001.txt", package = "rcprd"),
filetype = "observation", subset_patids = c(1,3,4,6), db = aurum_extract, overwrite = TRUE)
add_to_database(filepath = system.file("aurum_data", "aurum_allpatid_set1_extract_observation_002.txt", package = "rcprd"),
filetype = "observation", subset_patids = c(1,3,4,6), db = aurum_extract, append = TRUE)
add_to_database(filepath = system.file("aurum_data", "aurum_allpatid_set1_extract_observation_003.txt", package = "rcprd"),
filetype = "observation", subset_patids = c(1,3,4,6), db = aurum_extract, append = TRUE)We can then query this database using the db_query
function and return the first 3 rows. We will showcase how to use this
function to query the database for specific codes in section XXXX.
db_query utilises the package RSQLite, and more
details on how to query an SQLite database from within R is available in
RSQLite’s documentation (Müller et al.
2024).
db_query(db_open = aurum_extract, tab = "observation", n = 3)
#> patid consid pracid obsid obsdate enterdate staffid parentobsid
#> <char> <char> <int> <char> <Date> <Date> <char> <char>
#> 1: 1 33 1 100 1926-05-21 1967-04-13 79 95
#> 2: 1 66 1 46 1932-04-08 1928-04-19 34 17
#> 3: 1 41 1 53 1915-03-29 1994-03-21 35 79
#> medcodeid value numunitid obstypeid numrangelow numrangehigh
#> <char> <num> <int> <int> <num> <num>
#> 1: 498521000006119 48 16 20 28 86
#> 2: 401539014 22 1 2 27 8
#> 3: 13483031000006114 17 78 13 87 41
#> probobsid
#> <char>
#> 1: 54
#> 2: 35
#> 3: 74Next, the prescription data from the drugissue files is added to a
table called drugissue. A single SQLite database may
contain more than one table, so this data is added to a different table
within the same SQLite database. The table will take the same name as
the filetype argument, unless the table_name
argument is specified.
add_to_database(filepath = system.file("aurum_data", "aurum_allpatid_set1_extract_drugissue_001.txt", package = "rcprd"),
filetype = "drugissue", subset_patids = c(1,3,4,6), db = aurum_extract, overwrite = TRUE)
add_to_database(filepath = system.file("aurum_data", "aurum_allpatid_set1_extract_drugissue_002.txt", package = "rcprd"),
filetype = "drugissue", subset_patids = c(1,3,4,6), db = aurum_extract, append = TRUE)
add_to_database(filepath = system.file("aurum_data", "aurum_allpatid_set1_extract_drugissue_003.txt", package = "rcprd"),
filetype = "drugissue", subset_patids = c(1,3,4,6), db = aurum_extract, append = TRUE)This table can be queried in the same way, changing the
tab argument, which specifies the name of the table in the
SQLite database to query:
db_query(db_open = aurum_extract, tab = "drugissue", n = 3)
#> patid issueid pracid probobsid drugrecid issuedate enterdate staffid
#> <char> <char> <int> <char> <char> <Date> <Date> <char>
#> 1: 1 93 1 88 83 1925-11-15 1967-03-25 98
#> 2: 1 93 1 55 59 1933-07-12 1934-09-07 88
#> 3: 1 16 1 22 82 1946-03-31 1960-04-20 50
#> prodcodeid dosageid quantity quantunitid duration estnhscost
#> <char> <char> <num> <int> <int> <num>
#> 1: 3092241000033113 58 18 33 27 12
#> 2: 92041000033111 62 93 83 59 11
#> 3: 971241000033111 87 43 83 88 65Listing the tables in the SQLite database shows there are now two, named observation and drugissue.
RSQLite::dbListTables(aurum_extract)
#> [1] "drugissue" "observation"The add_to_database function allows specification of
filetype = c("observation", "drugissue", "referral", "problem", "consultation", "hes_primary","death"),
each corresponding to a specific function for reading in the
corresponding .txt files with correct formatting. The
"hes_primary" options correspond to the primary diagnoses
file in linked HES APC data. The "death" file corresponds
to the death file in the linked ONS data. If wanting to add other files
to the SQLite database, a user defined function for reading in the raw
.txt file can be specified through extract_txt_func. This
allows the user to add any .txt file to their SQLite database.
Finally, when manually adding files in this manner, it is good practice to close the connection to the SQLite database once finished.
RSQLite::dbDisconnect(aurum_extract)
3.2.2 Add all relevant
files to SQLite database using cprd_extract
In practice, there will be a high number of files to add to the
SQLite database and adding each one using add_to_database
would be cumbersome. We now repeat the extraction but using the
cprd_extract function, which is a wrapper for
add_to_database, and will add all the files in a specified
directory that contain a string matching the specified file type. Start
by creating a connection to the database:
aurum_extract <- connect_database(file.path(tempdir(), "temp.sqlite"))We then use cprd_extract to add all the observation
files into the SQLite database. If the connection
(aurum_extract) is to an existing database, which is the
case here, it will be overwritten when running
cprd_extract. The directory containing the files should be
specified using filepath. It will only read in and add
files with the text string specified in filetype in their
file name. The filetype argument takes values in
c("observation", "drugissue", "referral", "problem", "consultation").
We then query the first three rows of this database, and note they are
the same as previously.
### Extract data
cprd_extract(db = aurum_extract,
filepath = system.file("aurum_data", package = "rcprd"),
filetype = "observation", subset_patids = c(1,3,4,6), use_set = FALSE)
#> | | | 0%
#> Adding /home/runner/work/_temp/Library/rcprd/aurum_data/aurum_allpatid_set1_extract_observation_001.txt 2026-02-12 15:58:04.715758
#> | |======================= | 33%
#> Adding /home/runner/work/_temp/Library/rcprd/aurum_data/aurum_allpatid_set1_extract_observation_002.txt 2026-02-12 15:58:04.730528
#> | |=============================================== | 67%
#> Adding /home/runner/work/_temp/Library/rcprd/aurum_data/aurum_allpatid_set1_extract_observation_003.txt 2026-02-12 15:58:04.743339
#> | |======================================================================| 100%
### Query first three rows
db_query(db_open = aurum_extract, tab = "observation", n = 3)
#> patid consid pracid obsid obsdate enterdate staffid parentobsid
#> <char> <char> <int> <char> <Date> <Date> <char> <char>
#> 1: 1 33 1 100 1926-05-21 1967-04-13 79 95
#> 2: 1 66 1 46 1932-04-08 1928-04-19 34 17
#> 3: 1 41 1 53 1915-03-29 1994-03-21 35 79
#> medcodeid value numunitid obstypeid numrangelow numrangehigh
#> <char> <num> <int> <int> <num> <num>
#> 1: 498521000006119 48 16 20 28 86
#> 2: 401539014 22 1 2 27 8
#> 3: 13483031000006114 17 78 13 87 41
#> probobsid
#> <char>
#> 1: 54
#> 2: 35
#> 3: 74The process is then repeated for the drugissue files.
### Extract data
cprd_extract(db = aurum_extract,
filepath = system.file("aurum_data", package = "rcprd"),
filetype = "drugissue", subset_patids = c(1,3,4,6), use_set = FALSE)
#> | | | 0%
#> Adding /home/runner/work/_temp/Library/rcprd/aurum_data/aurum_allpatid_set1_extract_drugissue_001.txt 2026-02-12 15:58:04.772243
#> | |======================= | 33%
#> Adding /home/runner/work/_temp/Library/rcprd/aurum_data/aurum_allpatid_set1_extract_drugissue_002.txt 2026-02-12 15:58:04.786154
#> | |=============================================== | 67%
#> Adding /home/runner/work/_temp/Library/rcprd/aurum_data/aurum_allpatid_set1_extract_drugissue_003.txt 2026-02-12 15:58:04.797858
#> | |======================================================================| 100%
### List tables
RSQLite::dbListTables(aurum_extract)
#> [1] "drugissue" "observation"
### Query first three rows
db_query(db_open = aurum_extract, tab = "drugissue", n = 3)
#> patid issueid pracid probobsid drugrecid issuedate enterdate staffid
#> <char> <char> <int> <char> <char> <Date> <Date> <char>
#> 1: 1 93 1 88 83 1925-11-15 1967-03-25 98
#> 2: 1 93 1 55 59 1933-07-12 1934-09-07 88
#> 3: 1 16 1 22 82 1946-03-31 1960-04-20 50
#> prodcodeid dosageid quantity quantunitid duration estnhscost
#> <char> <char> <num> <int> <int> <num>
#> 1: 3092241000033113 58 18 33 27 12
#> 2: 92041000033111 62 93 83 59 11
#> 3: 971241000033111 87 43 83 88 65
### Disconnect
RSQLite::dbDisconnect(aurum_extract)The string in the file name to match on, function to read in the raw
data, and the name of the table in the SQLite database, can be altered
using the str_match, extract_txt_func and
table_name arguments respectively. Note the use of
str_match may be of particular importance if the naming
convention of the raw data differs from what we have described above.
The argument rm_duplicates = TRUE can be specified to
de-duplicate records before adding into the SQLite database. This will
increase computation time, and the derivation of many variables will not
be effected by having duplicate records, so consider carefully whether
it’s necessary to apply this step or not. There will also be the
opportunity to de-duplicate the records later on when querying the data.
Note that cprd_extract may run for a considerable period of
time when working with the entire CPRD AURUM database, and therefore it
is not recommended to run interactively. While creation of the SQLite
database may be time consuming, subsequent queries will be far more
efficient, so this is short term pain for a long term gain.
3.2.3 Add all relevant
files to SQLite database in a computationally efficient manner using the
set functionality.
When the number of patients in your cohort is very large (for example
millions, or tens of millions), the add_to_database
function may perform very slowly. This is because for each observation
in the file being added to the SQLite database,
add_to_database checks to see whether the patid is
contained in the vector subset_patids (a vector of length
20,000,000 in our case). We can utilise the structure of the CPRD AURUM
data to speed up this process. If data has the set naming
convention (see section 2.1), we know that we only need to search for
patids from subset_patids, that are in the corresponding
patient file. For example, when reading in file
aurum_allpatid_set1_extract_observation_00Y.txt (for any
Y), we only need to search whether patid is in the vector of
patids from subset.patid, that are also in
aurum_allpatid_set1_extract_patient_001.txt, which is much
smaller vector. This can reduce the computation time for
add_to_database and cprd_extract.
To achieve this, the subset_patids object should be a
data frame with two required columns. The first column should be
patid, the second should be set, reporting the
corresponding value of set which the patient belongs to. The first step
is therefore to create a patient file, which has an extra variable
set, the number following the text string set in
the patient file containing data for that patient. When reading in the
patient files to create a cohort, this can be done by specifying
set = TRUE. In this example, all individuals in our cohort
come from the file with string set1, and therefore this
variable is the same for all individuals in this cohort, however this
will not be the case in practice.
pat <- extract_cohort(filepath = system.file("aurum_data", package = "rcprd"), patids = as.character(c(1,3,4,6)), set = TRUE)
pat
#> patid pracid usualgpstaffid gender yob mob emis_ddate regstartdate
#> 1 1 49 6 2 1984 NA 1976-11-21 1940-07-24
#> 3 3 98 43 1 1930 NA 1972-06-01 1913-07-02
#> 4 4 53 72 2 1915 NA 1989-04-24 1969-07-11
#> 6 6 54 11 1 1914 NA 1926-09-09 1970-08-28
#> patienttypeid regenddate acceptable cprd_ddate set
#> 1 58 1996-08-25 1 1935-03-17 1
#> 3 81 1997-04-24 1 1912-04-27 1
#> 4 10 1951-09-05 0 1921-02-13 1
#> 6 85 1983-03-14 1 1963-08-27 1The patient file read in is the same as previously, with the addition
of the set column. This file can be reduced to just the
patid and set columns, and used as the input
to subset_patids when running the
add_to_database and cprd_extract functions.
When extracting data from observation files with set1 in the
name, it will only search for patient id’s with set == 1 in
the data.frame provided to subset_patids.
### Create connection to SQLite database
aurum_extract <- connect_database(file.path(tempdir(), "temp.sqlite"))
### Add observation files
cprd_extract(db = aurum_extract,
filepath = system.file("aurum_data", package = "rcprd"),
filetype = "observation",
subset_patids = pat,
use_set = TRUE)
#> | | | 0%
#> Adding /home/runner/work/_temp/Library/rcprd/aurum_data/aurum_allpatid_set1_extract_observation_001.txt 2026-02-12 15:58:04.836806
#> | |======================= | 33%
#> Adding /home/runner/work/_temp/Library/rcprd/aurum_data/aurum_allpatid_set1_extract_observation_002.txt 2026-02-12 15:58:04.850869
#> | |=============================================== | 67%
#> Adding /home/runner/work/_temp/Library/rcprd/aurum_data/aurum_allpatid_set1_extract_observation_003.txt 2026-02-12 15:58:04.86552
#> | |======================================================================| 100%
### Add drugissue files
cprd_extract(db = aurum_extract,
filepath = system.file("aurum_data", package = "rcprd"),
filetype = "drugissue",
subset_patids = pat,
use_set = TRUE)
#> | | | 0%
#> Adding /home/runner/work/_temp/Library/rcprd/aurum_data/aurum_allpatid_set1_extract_drugissue_001.txt 2026-02-12 15:58:04.878976
#> | |======================= | 33%
#> Adding /home/runner/work/_temp/Library/rcprd/aurum_data/aurum_allpatid_set1_extract_drugissue_002.txt 2026-02-12 15:58:04.892642
#> | |=============================================== | 67%
#> Adding /home/runner/work/_temp/Library/rcprd/aurum_data/aurum_allpatid_set1_extract_drugissue_003.txt 2026-02-12 15:58:04.904971
#> | |======================================================================| 100%
### Query first three rows of each table
db_query(db_open = aurum_extract, tab = "observation", n = 3)
#> patid consid pracid obsid obsdate enterdate staffid parentobsid
#> <char> <char> <int> <char> <Date> <Date> <char> <char>
#> 1: 1 33 1 100 1926-05-21 1967-04-13 79 95
#> 2: 1 66 1 46 1932-04-08 1928-04-19 34 17
#> 3: 1 41 1 53 1915-03-29 1994-03-21 35 79
#> medcodeid value numunitid obstypeid numrangelow numrangehigh
#> <char> <num> <int> <int> <num> <num>
#> 1: 498521000006119 48 16 20 28 86
#> 2: 401539014 22 1 2 27 8
#> 3: 13483031000006114 17 78 13 87 41
#> probobsid
#> <char>
#> 1: 54
#> 2: 35
#> 3: 74
db_query(db_open = aurum_extract, tab = "drugissue", n = 3)
#> patid issueid pracid probobsid drugrecid issuedate enterdate staffid
#> <char> <char> <int> <char> <char> <Date> <Date> <char>
#> 1: 1 93 1 88 83 1925-11-15 1967-03-25 98
#> 2: 1 93 1 55 59 1933-07-12 1934-09-07 88
#> 3: 1 16 1 22 82 1946-03-31 1960-04-20 50
#> prodcodeid dosageid quantity quantunitid duration estnhscost
#> <char> <char> <num> <int> <int> <num>
#> 1: 3092241000033113 58 18 33 27 12
#> 2: 92041000033111 62 93 83 59 11
#> 3: 971241000033111 87 43 83 88 65Note that there is no difference compared to the previously extracted SQLite databases. The computational gains from applying the subsetting in this manner will not be realised in this example. We do not close the connection, as we will now move onto querying the database to extract variables for creating an analysis-ready dataset.
3.3 Step 6: Querying the SQLite database to extract variables
Once the data has been extracted and stored in an SQLite database, it can now be queried to create variables of interest. Please note, if the answer to Q1 was yes, resulting in the implementation of steps 2 and 3, the process for querying the SQLite database in order to apply the exclusion criteria (step 3) will follow the exact same process as outlined here.
The normal process for extracting variables from electronic health
records is to create code lists, a group of codes which denote the same
condition. The database would then be queried for observations with
medical codes matching those in the code list. A variable
would then be defined based on this query. Whether this is a binary
variable, indicating whether an individual has any record of a given
code, or the most recent test result with the given code, or something
much more complex. In CPRD Aurum, medical diagnoses and tests are
identified from the observation file using medcodeids,
and prescription data is identified from the drugissue file using
prodcodeids. Creation of code lists is an important step of
data extraction, and we refer elsewhere for details on best practice for
developing code lists, and the limitations of working with code lists
(Williams et al. 2019, 2017; Watson et al. 2017;
Gulliford et al. 2009; Matthewman et al. 2024). The functions in
this section are split into three groups:
- Functions for extracting common variable types.
- Functions for extracting specific variables
- Functions for database queries and custom variable extraction
These functions extract and query the data relative to an index date. The index date may be a fixed date (e.g. 1st January 2010), a date which is different for each individual (e.g. date age 50 reached), or a combination of the two (e.g., maximum of 1st January 2010 and date aged 50 reached).
3.3.1 Functions for extracting common variable types
There are functions to extract three common variable types, history
of condition/medication prior to index date (extract_ho),
time from the index date until first occurrence of a medical
code/prescription or censoring (extract_time_until), and
most recent test result(s) in a given time frame and valid range
relative to the index date (extract_test_data). Variables
are calculated relative to the index date using the observation date
(obsdate) in the observation file and the issue date (issuedate) in the
drug issue file.
The first, extract_ho, extracts a binary variable based
on whether individual has a specified code recorded prior to index date.
This can be applied to search for history of medical diagnoses or
prescriptions. The index date ust be a variable in the cohort dataset,
and is specified through the indexdt argument.
### Define codelist
my_vector_codelist <- "187341000000114"
### Add an index date to cohort
pat$fup_start <- as.Date("01/01/2020", format = "%d/%m/%Y")
### Extract a history of type variable using extract_ho
ho <- extract_ho(cohort = pat,
codelist_vector = my_vector_codelist,
indexdt = "fup_start",
db_open = aurum_extract,
tab = "observation",
return_output = TRUE)
ho
#> patid ho
#> 1 1 0
#> 3 3 0
#> 4 4 0
#> 6 6 1The second is extract_time_until, which defines a
time-to-event/survival variable. This has two components, the time until
the first record of a specified code or censoring, and an indicator for
whether event was observed or censored. To derive a variable of this
type the cohort must also contain a time until censoring variable, which
can be specified through censdt.
### Add an censoring date to cohort
pat$fup_end <- as.Date("01/01/2024", format = "%d/%m/%Y")
### Extract a time until variable using extract_time_until
time_until <- extract_time_until(cohort = pat,
codelist_vector = my_vector_codelist,
indexdt = "fup_start",
censdt = "fup_end",
db_open = aurum_extract,
tab = "observation",
return_output = TRUE)
time_until
#> patid var_time var_indicator
#> 1 1 1461 0
#> 2 3 1461 0
#> 3 4 1461 0
#> 4 6 1461 0The third is extract_test, which will extract the most
recent test result in a given time frame. The number of days before and
after the index date to search for results are specified through
time_post and time_prev respectively. Test
results are identified from the observation file, using code lists.
Lower and upper bounds can also be specified for the extracted data
through lower_bound and upper_bound.
### Extract test data using extract_test_data
test_data <- extract_test_data(cohort = pat,
codelist_vector = my_vector_codelist,
indexdt = "fup_start",
db_open = aurum_extract,
time_post = 0,
time_prev = Inf,
return_output = TRUE)
test_data
#> patid value
#> 1 1 NA
#> 2 3 NA
#> 3 4 NA
#> 4 6 28More than one observation can be returned by specifying
numobs. Metadata of the test result, such as the unit of
measurement, date recorded, and the medical code, can be returned by
settings numunitid = TRUE. A variation of this function,
extract_test_data_var, will returns the standard deviation
of the test data within the specified time and value range. Once all the
variables of interest have been extracted, they can be merged into an
analysis-ready dataset (step 7).
### Recursive merge
analysis.ready.pat <- Reduce(function(df1, df2) merge(df1, df2, by = "patid", all.x = TRUE), list(pat[,c("patid", "gender", "yob")], ho, time_until, test_data))
analysis.ready.pat
#> patid gender yob ho var_time var_indicator value
#> 1 1 2 1984 0 1461 0 NA
#> 2 3 1 1930 0 1461 0 NA
#> 3 4 2 1915 0 1461 0 NA
#> 4 6 1 1914 1 1461 0 28The codelists can also be specified through an R
data.frame which must contain either a medcodeid
or prodcodeid column. This may allow the user to run
sensitivity analyses more easily if they would like to extract the same
variable for different subgroups of the same codelist. For example:
my_codelist_df <- data.frame("condition" = "mycondition", medcodeid = c("221511000000115", "187341000000114"), "subgroup" = c("subgroup1", "subgroup2"))
extract_test_data(cohort = pat,
codelist_df = subset(my_codelist_df, subgroup == "subgroup1"),
indexdt = "fup_start",
db_open = aurum_extract,
time_post = 0,
time_prev = Inf,
return_output = TRUE)
#> patid value condition medcodeid subgroup
#> 1 1 NA <NA> <NA> <NA>
#> 2 3 68 mycondition 221511000000115 subgroup1
#> 3 4 NA <NA> <NA> <NA>
#> 4 6 NA <NA> <NA> <NA>
extract_test_data(cohort = pat,
codelist_df = subset(my_codelist_df, subgroup == "subgroup2"),
indexdt = "fup_start",
db_open = aurum_extract,
time_post = 0,
time_prev = Inf,
return_output = TRUE)
#> patid value condition medcodeid subgroup
#> 1 1 NA <NA> <NA> <NA>
#> 2 3 NA <NA> <NA> <NA>
#> 3 4 NA <NA> <NA> <NA>
#> 4 6 28 mycondition 187341000000114 subgroup2Codelists can be specified in this way for any of the functions in
this section, or sections 3.3.2 and 3.3.3. However, the extra variable
from the codelist (i.e. the condition and
subgroup variables in the above example) will only be
returned in the output when it is meaningful to do so. For example, in
extract_ho, an individual may have many matching codes in
their medical history, and therefore it’s unclear which should be
returned.
3.3.2 Functions for extracting specific variables
There are also a number of functions that can be used to extract specific variables:
-
extract_bmi: Derives BMI scores. Requires specification of codelist for BMI, height, and weight separately. -
extract_cholhdl_ratio: Derives total cholesterol/high-density lipoprotein ratio. Requires specification of separate codelists for total cholesterol/high-density lipoprotein ratio, total cholesterol, and high-density lipoproteins separately. -
extract_diabetes: Derives a categorical variable for history of type 1 diabetes, history of type 2 diabetes or no history of diabetes. Requires specification of separate codelists for type 1 and type 2 diabetes. Individuals with codes for both are designated as type 1. -
extract_smoking: Derives a categorical variable for smoking status. Requires specification of seperate codelists for non-smoker, ex-smoker, light smoker, moderate smoker and heavy smoker. If the most recent smoking status is non-smoker, but there are historical codes which indicate smoking, then individual will be classified as an ex-smoker.
It was deemed that these variables required custom functions because
their definitions did not fit into any of the variable types from
section 3.3.1. In each case, a number of steps are taken in order to
clean or manipulate the data in order to get the desired output. For
example, height measurements recorded in centimeters are converted to
metres in order to calculate BMI scores. This is done through the use of
the numunitid variable in the observation file. For both
BMI and cholesterol/high-density lipoprotein ratio, the variable can be
either be identified directly, or calculated from the component
measures. In each case, the component parts must be recorded in the
specified time range relative to the index date. For smoking status, if
an individuals most recent medical observation was recorded as a
non-smoker, but their medical record shows previous smoking, the most
recent record is changed to ex-smoker. For diabetes status, diabetes if
often recorded with generic codes such as “diabetes mellitus”, which
does not specify which type. This is dealt with by assuming all generic
codes refer to type 2 diabetes, unless that individual also has a
specific type 1 diabetes code, in which case they will be determined to
have type 1 diabetes as opposed to type 2.
The full details on extracting these variables are provided in the vignette titled Details-on-algorithms-for-extracting-specific-variables. However, it is important to state, that the correct way to define a variable may change from study to study. Therefore when using these functions to extract variables, we encourage taking the time to ensure that the way the variable is extracted matches the definition in ones study, and edit these functions and algorithms accordingly.
3.3.3 Functions for database queries and custom variable extraction
These functions are utilised internally in the functions from sections 3.3.1 and 3.3.2. They have been provided to more easily enable package users to write their own functions for extracting variables that are not covered in the previous two sections.
The db_query function will query the SQLite database for
observations where the medcodeid or prodcodeid is in a
specified codelist. For example, we can query the observation
table for all codes with medcodeid of 114311000006111 Setting
rm_duplicates = TRUE will de-duplicate the output. If the
codelist is specified through an R data.frame with the
codelist_df argument, the returned query will also contain
the variables from the codelist data.frame.
my_db_query <- db_query(db_open = aurum_extract,
tab ="observation",
codelist_vector = "114311000006111")
my_db_query
#> patid consid pracid obsid obsdate enterdate staffid parentobsid
#> <char> <char> <int> <char> <Date> <Date> <char> <char>
#> 1: 1 41 1 100 1904-09-27 1966-12-02 33 39
#> 2: 3 34 1 79 1976-07-31 1979-03-13 25 90
#> 3: 3 79 1 18 1947-09-30 1927-10-31 93 55
#> 4: 3 7 1 22 1989-07-24 1914-11-30 26 63
#> 5: 4 42 1 43 1924-09-03 1955-03-13 52 93
#> 6: 4 80 1 80 1945-11-26 1993-03-28 48 25
#> 7: 4 43 1 64 1909-09-25 1931-07-17 84 44
#> 8: 6 49 1 96 1932-05-29 1947-10-06 60 86
#> 9: 6 42 1 68 1931-05-20 1978-09-24 81 59
#> medcodeid value numunitid obstypeid numrangelow numrangehigh probobsid
#> <char> <num> <int> <int> <num> <num> <char>
#> 1: 114311000006111 18 29 78 4 89 61
#> 2: 114311000006111 83 69 56 75 71 84
#> 3: 114311000006111 85 43 9 61 8 84
#> 4: 114311000006111 16 99 13 64 65 54
#> 5: 114311000006111 59 73 61 89 53 13
#> 6: 114311000006111 88 91 66 20 42 3
#> 7: 114311000006111 100 9 29 71 93 90
#> 8: 114311000006111 15 47 77 26 82 16
#> 9: 114311000006111 81 61 32 67 20 45The combine_query_boolean function will then assess
whether each individual in a specified cohort (pat) has an
observation in the queried data (obtained using db_query)
within a specified time frame from the index date, returning a 0/1
vector. The cohort must contain a variable called
indexdt containing the index date. This function is useful
when defining ‘history of’ type variables, where we want to know if
there is any record of a given condition prior to the index date.
### Add an index date to pat
pat$indexdt <- as.Date("01/01/2020", format = "%d/%m/%Y")
### Combine query with cohort creating a boolean variable denoting 'history of'
combine.query.boolean <- combine_query_boolean(cohort = pat,
db_query = my_db_query,
query_type = "med")
combine.query.boolean
#> [1] 0 1 1 1The combine_query function will merge a cohort with the
queried data and return a specified number of observations
(numobs) within a specified time frame from the index date.
This is useful when extracting test data and requiring access to the
values of the tests, or when specifying variables that require > 1
observation within a certain time frame (i.e. two prescriptions within a
month prior to index date). Setting reduce_output = TRUE
will remove the majority of variables from the merged datasets. For
queries from the observation table, the query type can be
specified as "med" or "test". Inputting
query_type = "med" will just return the date of the
observations and the medcodeid.
### Combine query with cohort retaining most recent three records
combine.query <- combine_query(cohort = pat,
db_query = my_db_query,
query_type = "med",
numobs = 3,
reduce_output = TRUE)
combine.query
#> patid medcodeid obsdate
#> <char> <char> <Date>
#> 1: 3 114311000006111 1976-07-31
#> 2: 3 114311000006111 1947-09-30
#> 3: 3 114311000006111 1989-07-24
#> 4: 4 114311000006111 1924-09-03
#> 5: 4 114311000006111 1945-11-26
#> 6: 6 114311000006111 1932-05-29
#> 7: 6 114311000006111 1931-05-20For query_type = "test", the value and
other relevant information will also be returned, and those with NA
values removed (although this can be altered through argument
value_na_rm).We then close the connection to the
database.
### Extract a history of type variable using extract_ho
combine.query <- combine_query(cohort = pat,
db_query = my_db_query,
query_type = "test",
numobs = 3,
reduce_output = TRUE)
combine.query
#> patid medcodeid obsdate value numunitid numrangelow numrangehigh
#> <char> <char> <Date> <num> <int> <num> <num>
#> 1: 3 114311000006111 1976-07-31 83 69 75 71
#> 2: 3 114311000006111 1947-09-30 85 43 61 8
#> 3: 3 114311000006111 1989-07-24 16 99 64 65
#> 4: 4 114311000006111 1924-09-03 59 73 89 53
#> 5: 4 114311000006111 1945-11-26 88 91 20 42
#> 6: 6 114311000006111 1932-05-29 15 47 26 82
#> 7: 6 114311000006111 1931-05-20 81 61 67 20
### Disconnect
RSQLite::dbDisconnect(aurum_extract)If the query was from the drugissue table, then
query_type = "drug" should be specified, and the date of
the observations and the prodcodeid will be returned. If
reduce_output = FALSE, no variables will be removed the
output. The functions in this section can be used as building blocks to
extract desired variables (e.g. see functions in section 3.3.2).
3.3.4 Saving extracted variables directly to a disk drive, and utilising rAURUMs suggested directory system
So far all extracted variables (using functions from section 3.3.1
and 3.3.2) have been read into the R workspace by specifying
return_output = TRUE. When working with large cohorts it
may be preferable to save the output directly onto a disk drive, by
specifying out_save_disk = TRUE. The file path to save the
output can be specified manually through the out_filepath
argument. However, if this argument is left as NULL,
rcprd will attempt to save the extracted variable into
a directory “data/extraction/” relative to the working directory. The
name of the file itself will be dependent on the variable name specified
through argument varname. This can be a very convenient way
to save the output directly to disk without having to repeatedly specify
file paths and file names.
There is similar functionality when specifying the codelists.
Codelists can be specified in two ways. The first is to read the
codelist into R as a character vector and then specify through the
argument codelist_vector, which has been done in all the
previous examples. Alternatively, codelists stored on the disk drive can
be referred to from the codelist argument in many
rcprd functions, but requires a specific underlying
directory structure. The codelist on the disk drive must be stored in a
directory called “codelists/analysis/” relative to the working
directory. The codelist must be a .csv file, and contain a column
medcodeid, prodcodeid or ICD10 depending on
the table being queried. The input to argument codelist
should just be a character string of the name of the files (excluding
the suffix ‘.csv’). The codelist_vector argument will take
precedence over the codelist argument if both are
specified.
Finally, there is similar functionality for accessing the SQLite
database internally, rather than having to 1) open a connection, 2) use
this as an input in the functions, and then 3) remember to close the
connection. Instead, if the SQLite database is stored in a directory
“data/sql/” relative to the working directory, the SQLite database can
be referred to by name (a character string) with the argument
db. A connection to the SQLite datbase will be opened
internally within the function call, the SQLite database will be
queried, and then the connection closed. Alternatively, a SQLite
database stored anywhere on the disk drive can be accessed by specifying
the full filepath (character string) with the argument
db_filepath.
This workflow is advantageous as it avoids hard file paths which
beneficial if wanting to move your code onto another computer system.
Furthermore, once codelists and the SQLite database have been created
and stored in the appropriate folders, they can simply be referred to by
name, resulting in an easier workflow. The function
create_directory_system() will create the directory system
required to use rcprd in this way. To avoid repetition
of the previous section, this is showcased just once using the
extract_ho function. For the sake of this example, we start
by setting the working directory to a directory called
inst/example within rcprd. To maintain the new
working directory across multiple R markdown code chunks, we use
knitr::opts_knit$set. To follow this section, the user
should simply set their working directory as usual using
setwd().
Next, the create_directory_system() function can be used
to generate the required directory structure.
suppressMessages(
create_directory_system()
)
file.exists(file.path(tempdir(), "data"))
#> [1] TRUE
file.exists(file.path(tempdir(), "codelists"))
#> [1] TRUE
file.exists(file.path(tempdir(), "code"))
#> [1] TRUEAn SQLite database called “mydb.sqlite” is then created in the “data/sql” directory, using the same data from the previous examples:
## Open connection
aurum_extract <- connect_database("data/sql/mydb.sqlite")
## Add data to SQLite database using cprd_extract
cprd_extract(db = aurum_extract,
filepath = system.file("aurum_data", package = "rcprd"),
filetype = "observation", use_set = FALSE)
#> | | | 0%
#> Adding /home/runner/work/_temp/Library/rcprd/aurum_data/aurum_allpatid_set1_extract_observation_001.txt 2026-02-12 15:58:05.473439
#> | |======================= | 33%
#> Adding /home/runner/work/_temp/Library/rcprd/aurum_data/aurum_allpatid_set1_extract_observation_002.txt 2026-02-12 15:58:05.491334
#> | |=============================================== | 67%
#> Adding /home/runner/work/_temp/Library/rcprd/aurum_data/aurum_allpatid_set1_extract_observation_003.txt 2026-02-12 15:58:05.503114
#> | |======================================================================| 100%
## Disconnect
RSQLite::dbDisconnect(aurum_extract)Finally, a code list called mylist.csv is created and saved into the codelists/analysis/ directory.
### Define codelist
my_codelist <- data.frame(medcodeid = "187341000000114")
### Save codelist
write.csv(my_codelist, "codelists/analysis/mylist.csv")The mydb.sqlite database can now be queried to create a ‘history of’ type variable using the codelist mylist.csv, with the output saved directly onto the disk drive.
extract_ho(cohort = pat,
codelist = "mylist",
indexdt = "fup_start",
db = "mydb",
tab = "observation",
return_output = FALSE,
out_save_disk = TRUE)Note that in order to run extract_ho here, a connection
to the SQLite database did not need to be created, the codelist did not
need to be in the R workspace, and there is no output from this
function. Instead the extracted variable has been saved onto the disk
drive in an .rds file, and can be read in using:
readRDS("data/extraction/var_ho.rds")
#> patid ho
#> 1 1 0
#> 3 3 0
#> 4 4 0
#> 6 6 1This setup can be used in conjunction with any of the functions from
step 6 (i.e. extract_test_var,
extract_time_until or db_query).
3.3.5 Extracting longitudinal data/time varying covariates
All of the functions in section 3.3.1 and 3.3.2 have the option to
extract data at a given time point post index date (specified through
the t argument). This allows users to extract data at fixed
intervals, which can be utilised for longitudinal analyses where
time-varying covariates are required. If saving the extracted variables
directly to the disk drive (out_save_disk = TRUE), the time
at which data was extracted from, t, will be added to the
file name by default.
3.3.6 Working with linked data
This worked example has overlooked how to work with linked data.
Linked data can be added to the SQLite database. The primary diagnosis
file can be added using the add_to_database() function by specifying
filetype = “hes_primary”. Any other linked file can be added to the
SQLite database by writing a user-defined function which reads in the
text file and formats the variables appropriately, and specifying this
through the extract_txt_func argument. However, the user
will have to define functions for querying the linked data and creating
variables for analysis. Working with linked data has not been made part
of the core functionality because it is often much smaller in size, and
files (e.g. HES Admitted Patient Care primary diagnosis file, or Office
for National Statistics death registration data) are not broken up into
large number of smaller files. This means they can be more easily read
into R and dealt with in the R workspace.
4 Discussion
rcprd is an R package which allows users to process CPRD Aurum data in R in a consistent and computationally efficiency manner. It provides functionality to both read in and store data, and create analysis-ready datasets. The process avoids reading thousands of raw text files into the R workspace whenever a variable needs to be derived, minimising the risk of coding errors. rcprd enables the handling and storing the raw data, achieved through the creation of an SQLite database using RSQLite. The user can define their own functions for reading in the raw data, allowing these functions to be applied to other electronic health records, or future versions of CPRD Aurum which have different data structures. The functions for extraction of variables to create analysis-ready datasets involve are split into three groups: 1) Functions for extracting common variable types (history of a specified condition, time until event occurs, or most recent test result); 2) Functions for extracting specific variables; 3) Functions for database queries and custom variable extraction. These querying large data files that could not otherwise be handled in the R workspace. These functions uses computationally efficient SQL queries to query large datasets that could not be read into the R workspace, but no-user knowledge of SQL is required.
By utilising RSQLite for the storing and querying of the raw data, rcprd follows the suggested approach of rEHR (Springate et al. 2017). In many ways, rEHR is more comprehensive than rcprd, as it could also be used for case-control matching, cutting up a survival cohort by time-varying covariates, and constructing clinical code lists. Both packages provide functionality to query the underlying database for observations with specific medical or prescription codes without needing SQL experience, however differ in their method for doing so. rEHR functions return observations between specified dates, whether that is all clinical codes, or the first/last clinical code in that period. These functions can also be applied across multiple time periods (i.e. by year) simultaneously. In contrast, rcprd functions query the database and return observations in a time period relative to an index date, which may (or may not) be a different date for each patient. As well as functions to query the database, rcprd also provides functions which will extract specific variable types, again relative to a given index date. For example, a binary variable based on existence of a clinical code prior to the index date, a test result between a specified upper and lower bound, or a time-to-event/survival type variable. These functions can also be applied any number of days before/after the specified index date to allow extraction of data for longitudinal analyses. The approach of rcprd, extracting variables relative to an index date is common when building datasets to be used for development or validation of a clinical prediction model,(Riley et al. 2019) whereas the functions contained in rEHR are relevant for a wider range of epidemiological analyses, including case-control studies and reporting descriptive properties such as incidence/prevalence.
aurumpipeline takes a different approach to rEHR and rcprd by using parquet files to store the data as opposed to SQLite. Parquet files are efficient for data storage and are optimised for query performance, meaning this setup has a high ceiling in term of computational efficiency. aurumpipeline provides functions to query the raw data between two fixed dates, with the option to define a binary variable depending if specified medical codes are recorded in this time period. Beyond this, the arrow (Richardson et al. 2024) package is recommended for any further data base queries, meaning the derivation of other variables types will require user-developed functions.
The strength of rcprd is to simplify the complex process of turning raw CPRD data into an analysis-ready dataset, and does this by following the process of (Springate et al. 2017). Functions for extracting variables have been designed to be user friendly, to the extent that all that needs to be specified is the index date and code list, and a number of different common variable types can be derived. More basic functions are also provided, which simply return queries of the underlying data, in order to allow the user flexibility in defining their own functions for extracting other variables or summary statistics. The main limitation of this package is one inherent with all R packages, that they must be continuously maintained as R is updated. This is the main reason rcprd has been developed, in light of the archiving of rEHR. Package rights will be set-up so that rcprd can be maintained and taken over by other individuals. Another limitation is that the scope of this package is not comprehensive, for example in comparison to rEHR, and may not cover the needs of all statisticians/epidemiologists. However, as the scope and size of the package increases, so does the task of maintaining it. We believe in it’s current state, maintenance of rcprd is manageable going forwards. Furthermore, rcprd provides the foundations to build a data set for any type of analysis, some tasks will just require more user-input in order to define new functions around the database queries.
In summary, the main goal of this package is to reduce the duplication of time and effort among those using CPRD data for their research, allowing more time to be focused on other aspects of research projects. rcprd will be actively maintained for the foreseeable future. Suggestions for improvement are encouraged and can be posted on GitHub: https://github.com/alexpate30/calibmsm.
A mirror of this vignette has been published on August 19th 2025 (Pate et al. 2025)